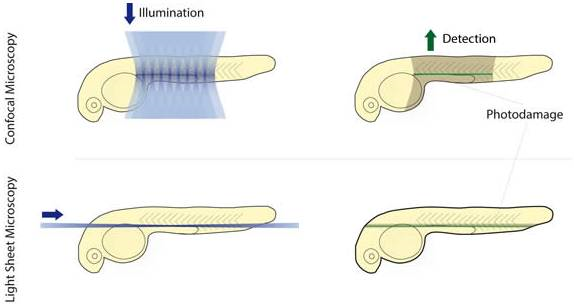
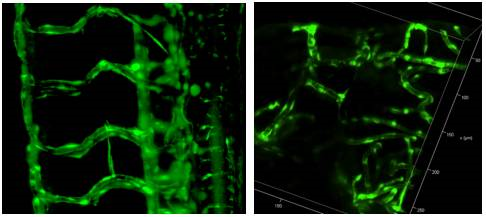
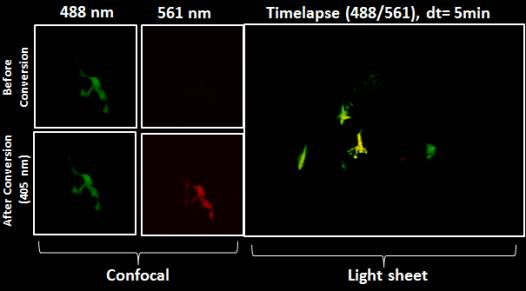
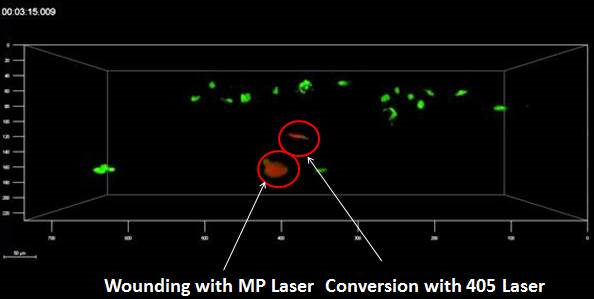
The concept of Light Sheet Fluorescence Microscopy (LSFM) was born in 1903, but it has not developed much since then. In the 1990s, the Francis Spelman Laboratory at the University of Washington developed a series of experimental methods for the quantitative measurement of the structure of mouse hair cells and other characteristics of the cochlea. Inspired by previous researchers using lateral light illumination to observe the surface structure, laboratory researchers invented the orthogonal plane fluorescence optical sectioning (OPFOS) and obtained the clear fluorescence image of the entire cochlea for the first time. (1) (2) (3). In 2004, the publication of the SPIM (Single plane illumination microscopy) article greatly promoted the development and use of light microscopy (4). The article emphasizes its practicability for embryonic development research and gives the plaque of the scorpion ganglion cell. And fluorescent images of long-term imaging of Drosophila embryo development. In 2010, at the first Symposium on Fluorescence Microscopy, the researchers decided to use LSFM as the uniform name for this type of microscope (5). Subsequent appearances of many forms of light microscopy, such as scanning light (6), two-photon scanning (7) and bessel beam (8), lattice (9) and other new light microscopy. These methods continue to improve the imaging resolution, penetration depth and imaging field of the light microscope to meet the needs of biological development. Photomicrograph system imaging principle The light microscopy system uses a beam of light to excite fluorescent samples from the side of the sample, using CCD or SCMOS for detection, and the illumination path and the fluorescence detection path are perpendicular to each other. Since the plane in which the sample is excited is the imaging plane, there is no defocus excitation, and optical slicing can be automatically obtained, thereby minimizing photobleaching and optical damage. The light microscopy system uses CCD or SCMOS imaging, the speed is usually tens of frames per second, or even hundreds of frames, so by moving the sample under the light sheet to make the incident beam excited to different planes, the whole can be easily and very quickly obtained. The traditional confocal excitation and detection of the 3D image of the tissue is in the same direction, the entire illumination area is in an excited state, and the undetected area is easily quenched after a long period of illumination, and the recording of several days cannot be guaranteed; Imaging with point scans is relatively slow. The difference between light microscope and confocal microscope In 2015, Leica introduced its own light film system, which uses a unique TwinFlect mirror to allow the excitation beam to be incident on the sample from both left and right directions, with uniform illumination, ensuring cell-level resolution. The fast imaging speed, high resolution and low phototoxicity allow the sample to retain its biological activity in the system, completing long-lived biological culture and imaging for hours or even days. In addition, the Leica light system is based on confocal and can be used in conjunction with confocal microscopy to perform operations such as light activation, light conversion, and subsequent tracking. Common applications for Leica light microscopy systems Rapid three-dimensional imaging of embryos and small organisms (such as model animals zebrafish, nematodes, fruit flies, etc., plant Arabidopsis, etc.), such as: cell migration, heart and vascular development, neurodevelopment, etc. Real-time imaging of three-dimensional cell culture, spheres and cysts, tissue culture, and organ culture. Combining confocal or two-photon laser microscopy, the optical stimulation and tracking functions are completed, and the observation and experimental methods are more flexible. Fast 3D imaging Zebrafish vascular system 3D reconstruction 2. Fast, long-term 3D imaging of the living body, capturing the entire dynamic process High-speed imaging: the heart of a zebrafish beating Drosophila back closure process 3. The only system that can combine confocal and light film for follow-up of optical operation After the zebrafish neuron light conversion (Kaede), the movement of the cell is continuously recorded. The zebrafish tail, after light conversion (Kaede, 405 nm), repairs the damaged part caused by the multiphoton laser Tracking light-converted cells using LAS X 3D analysis Long-term image acquisition needs to maintain sample activity, and the Leica light system can be loaded with an incubation device to provide good growth conditions and maintain activity. In addition, the entire system is highly open and has more room to operate. references 1. Voie AH, Burns DH, Spelman FA. (1993) Orthogonal-plane fluorescence optical sectioning: three-dimensional imaging of macroscopic biological specimens. J Microsc. Jun;170(Pt 3):229-36. 2. Voie AH, Spelman FA. (1995) Three-dimensional reconstruction of the cochlea from two-dimensional images of optical sections. Comput Med Imaging Graph. Sep-Oct;19(5):377-84. 3. Voie AH. (2002) Imaging the intact guinea pig tympanic bulla by orthogonal-plane fluorescence optical sectioning microscopy. Hear Res. Sep;171(1-2):119-128. Erratum in: Hear Res. 2003 Jul;181 (1-2): 144. 4. Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J., and Stelzer, EH (2004) Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science (New York, NY 305, 1007-1009 5. Reynaud EG, Tomancak P. (2010) Meetingreport: first light sheet based fluorescence microscopy workshop. Biotechnol J. Aug; 5(8): 798-804. doi: 10.1002/biot.201000177 6. Keller, PJ, Schmidt, AD, Wittbrodt, J., and Stelzer, EH (2008) Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science (New York, NY 322, 1065-1069 7. Truong, TV, Supatto, W., Koos, DS, Choi, JM, and Fraser, SE (2011) Deep and fast live imaging with two-photon scanned light-sheet microscopy. Nature methods 8, 757-760 8. Planchon, TA, Gao, L., Milkie, DE, Davidson, MW, Galbraith, JA, Galbraith, CG, and Betzig, E. (2011) Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nature methods 8, 417-423 9. Chen, BC, Legant, WR, Wang, K., Shao, L., Milkie, DE, Davidson, MW, Janetopoulos, C., Wu, XS, Hammer, JA, 3rd, Liu, Z., English, BP, Mimori-Kiyosue, Y., Romero, DP, Ritter, AT, Lippincott-Schwartz, J., Fritz-Laylin, L., Mullins, RD, Mitchell, DM, Bembenek, JN, Reymann, AC, Bohme, R ., Grill, SW, Wang, JT, Seydoux, G., Tulu, US, Kiehart, DP, and Betzig, E. (2014) Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science (New York , NY 346, 1257998 Mobile Flipchart U Stand,Magnetic Whiteboard Sheets For Board,Magnetic Glass Flipchart Easel Board,Magnetic Flipchart Easel Board Dongguan Aoxing Audio Visual Equipment CO.,Ltd , https://www.aoxing-whiteboard.com